
MYNX CONTROL Vascular Closure Device
The outstanding design and predictable deployment of MYNX CONTROL™ Vascular Closure Device delivers outstanding performance and control, for reliable secure arterial closures.*
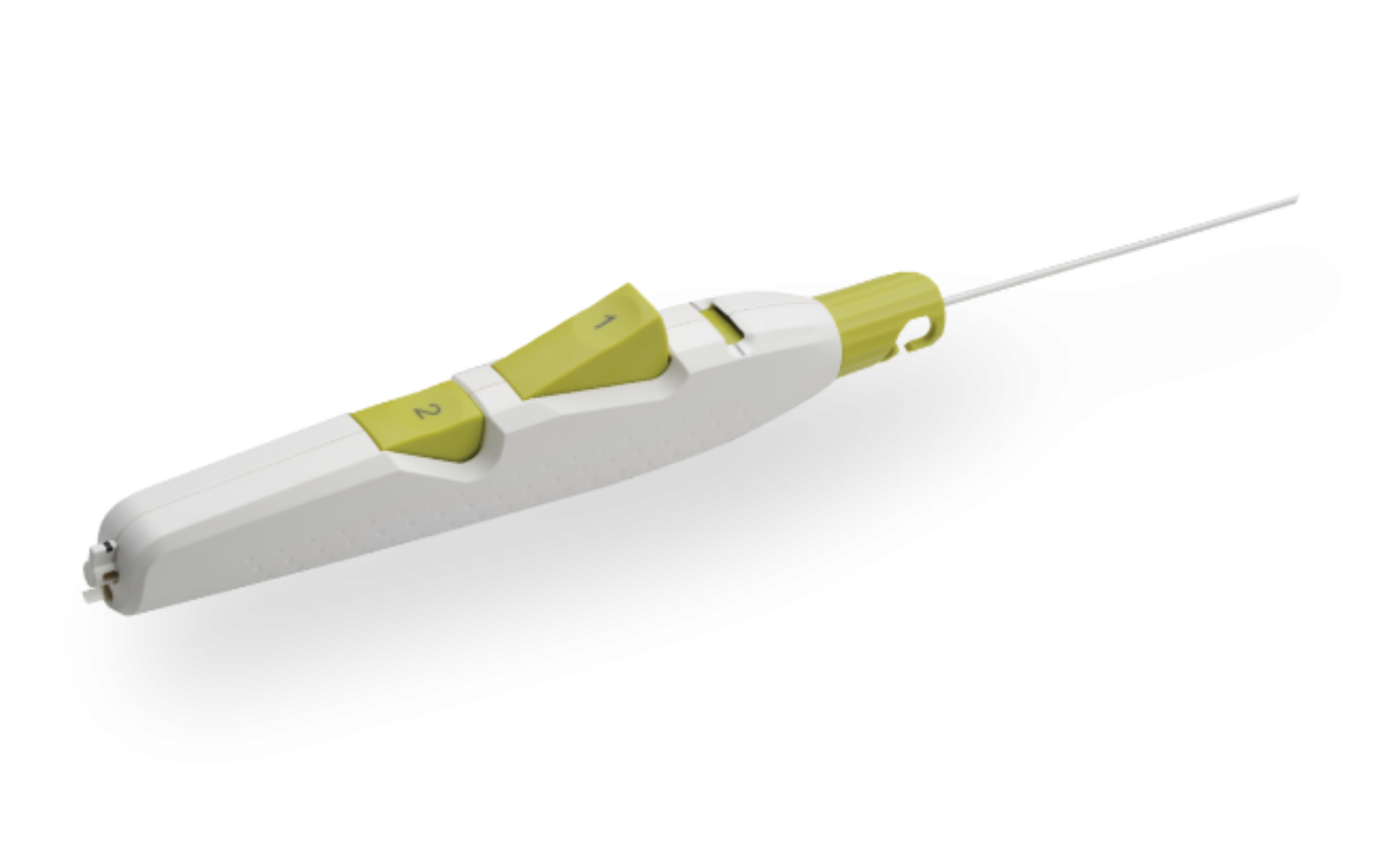
Product Description
MYNX CONTROL Vascular Closure Device integrates active extravascular sealing and resorbability properties with a next-generation delivery system to maximize predictability, safety, and ease of use in sealing 5-7F femoral arterial access sites.
Featuring:
- A next-generation deployment system designed for predictability and ease of use with a 2-button streamline procedural steps
- A sheath catch compatible with the procedural sheath
- A tension indicator providing visual confirmation of device position for proper sealant deployment
- Available in 5F, 6F, and 7F sizes
Resources
Please refer to Cordis Product Catalogue for complete product information.
MYNX CONTROL™ Vascular Closure Device - Catheter Lab Video
MYNX CONTROL™ Vascular Closure Device - Procedure Animation
The MYNX CONTROL™ VCD includes:
- (1) MYNX CONTROL™ VCD including balloon catheter and integrated polyethylene glycol sealant
- (1) 10 ml locking syringe
| Product | Size | Order Number |
MYNX CONTROL™ VCD | 5F | MX5060E |
| MYNX CONTROL™ VCD | 6F / 7F | MX6760E |
*Order Numbers are only valid for Europe, Middle East, and Africa.
IFU & Safety
Please refer to the Instructions for Use for complete information, including indications, precautions, warnings, and potential adverse events.
To request product specific Summary of Safety and Clinical Performance (SSCP), please email CordisMedInfo@cordis.com.
Customer Service and Ordering Information
For country-specific contact details, please see this page.

