SELUTION SLR PTA Drug Eluting Balloon
The SELUTION SLR™ Drug-Eluting Balloon (DEB) is the next step in the evolution of Leave Nothing Behind with Sustained Limus Release.
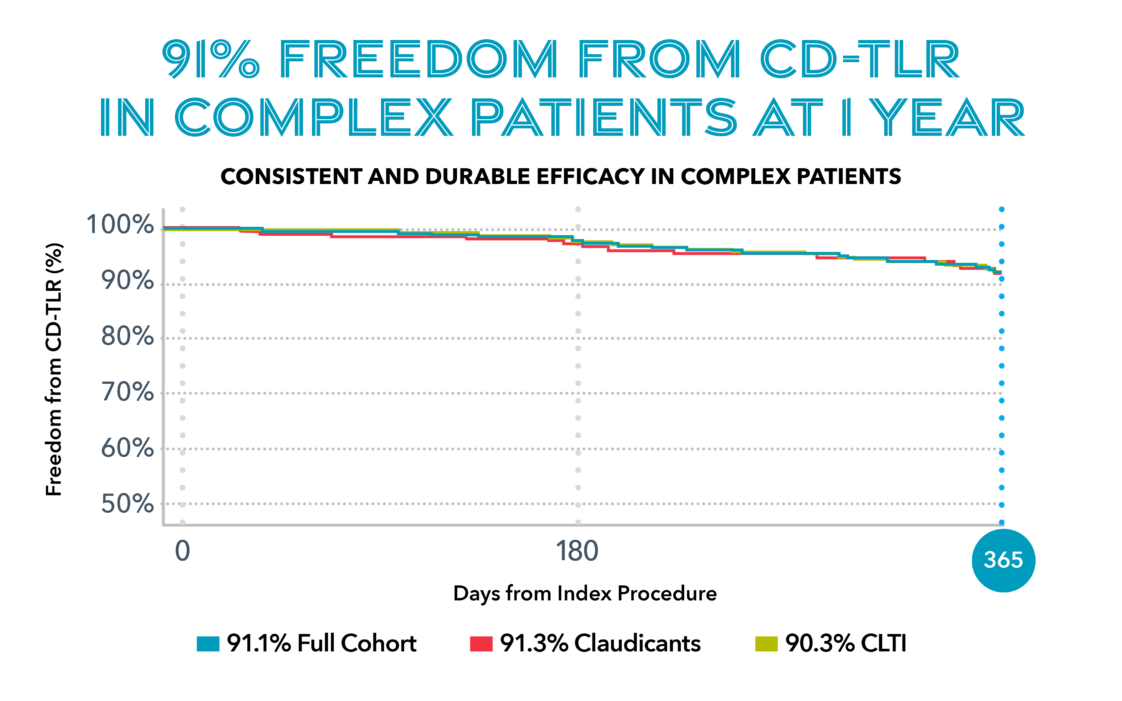
Real-World Evidence. Proven Results
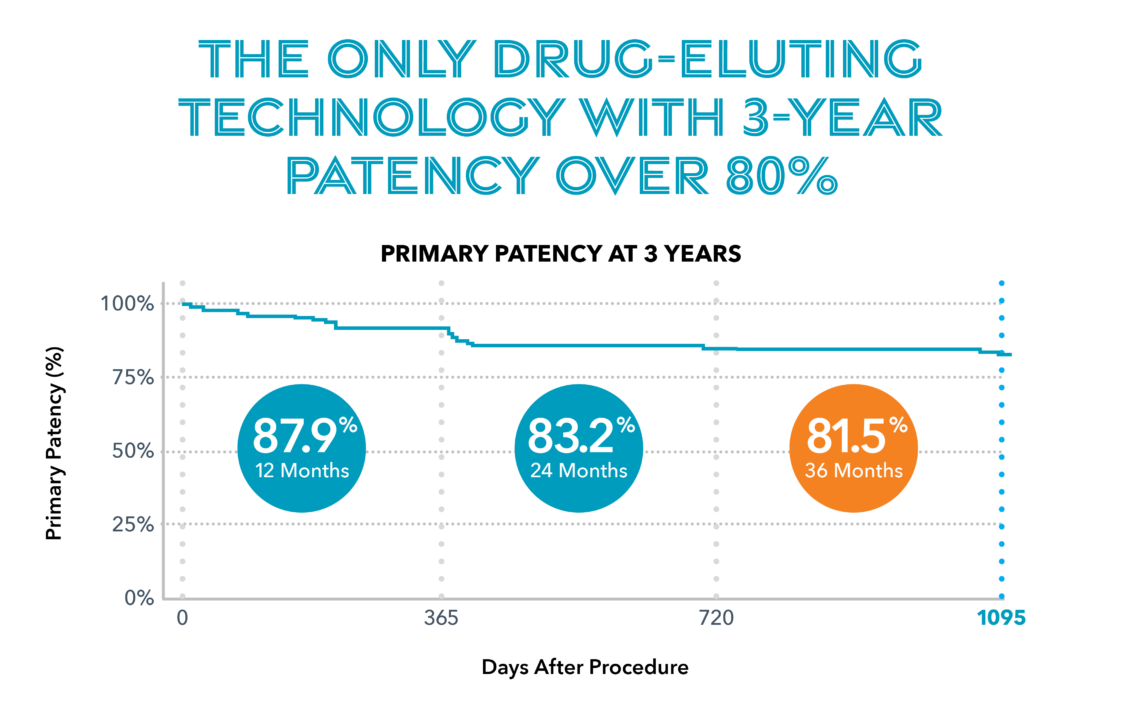
The New Paradigm for Peripheral Interventions
Breakthrough Technology1 to deliver sustained limus release.
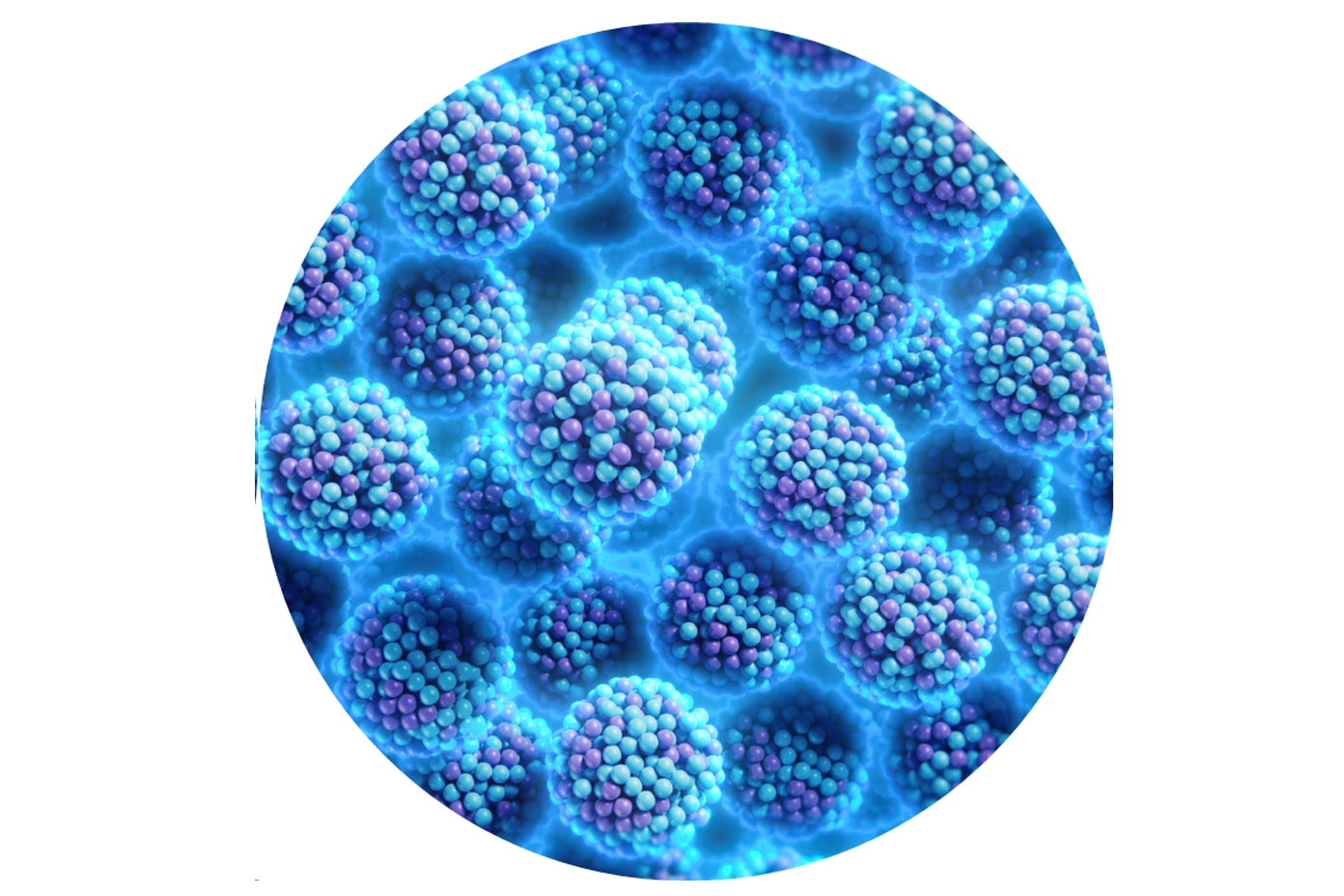
PHOSPHOLIPID COATING
A phospholipid blend containing and protecting MicroReservoirs, each incorporated with a 1 μg/mm² Sirolimus dose
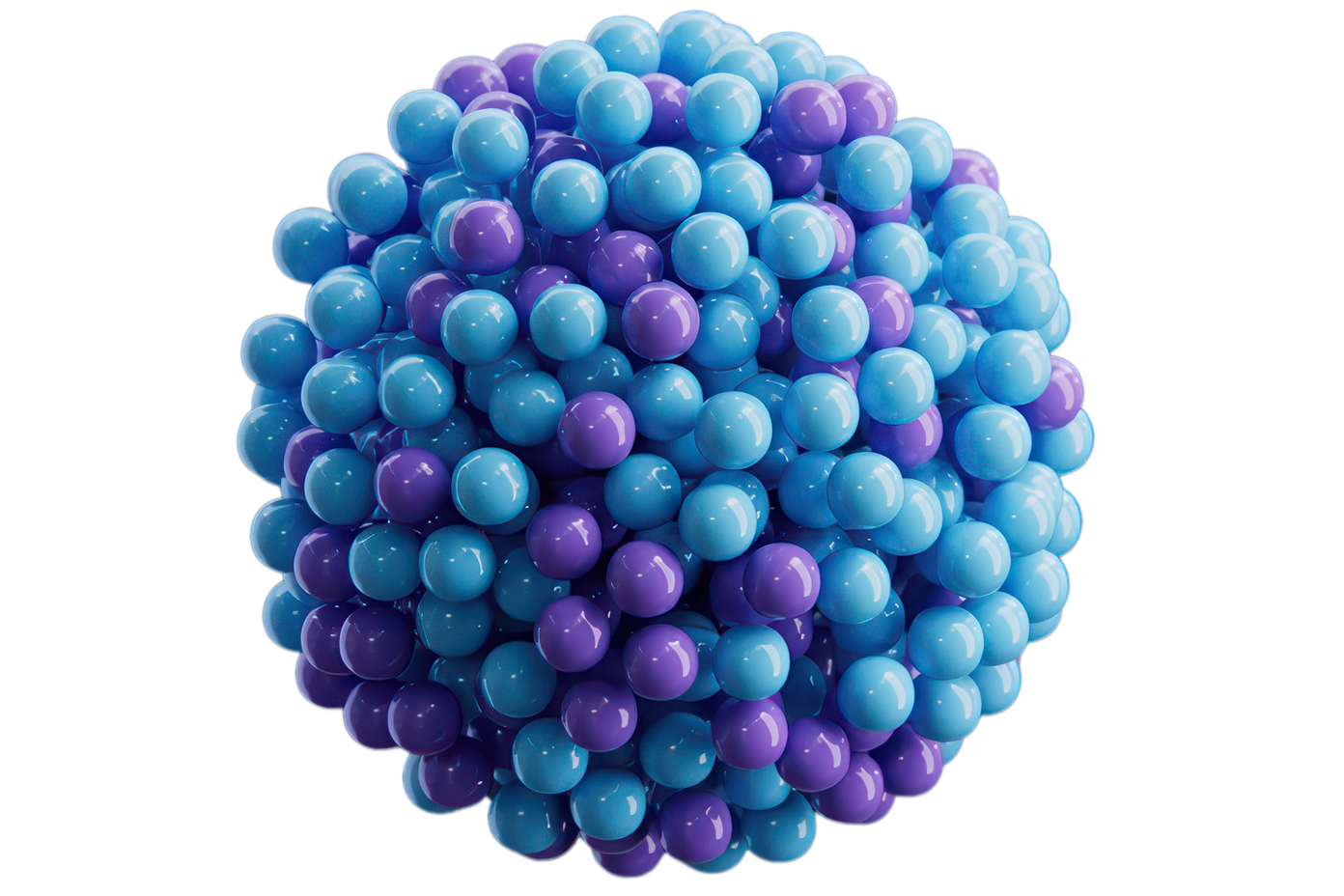
MICRORESERVOIRS
With a single intervention, millions of MicroReservoirs deliver a sustained and consistent drug release at a therapeutic level.
1. SELUTION SLR DEB was the first Sirolimus Drug-Eluting Balloon, for BTK, to be awarded Breakthrough Device designation by the US Food and Drug Administration.

HOMOGENEOUS IN-TISSUE DISTRIBUTION
No drug concentration peaks and vacancies as is typical of crystal formulations
Advanced Coating Technology

Better Uniformity and Smaller Particle Size2
| Proprietary Phospholipid Coating contains and protects microreservoirs. The coating is transferred to the vessel wall |
| MicroReservoirs (~4 μm) are as small or smaller than red blood cells |
| No local toxic effect on distal tissues/organs |
2. Med Alliance 2024 Data on file.
Dr. Palena BTK CLI and Drug Eluting Balloons
Dr. Palena's (Italy) case review of BTK CLI treatment with SELUTION SLR PTA Balloons.
Prof. Rammos SFA and BTK with Limus-Eluting Balloons
Prof. Rammos (Germany) discusses the use of Limus-eluting ballons for SFA and BTK lesions.
Dr. Lichtenberg - SUCCESS 6-month Results
Dr. Lichtenberg discusses the results of the SUCCESS six-month all-comers registry at CIRSE 2024 after the late-breaking results were presented in the First @CIRSE session
Advancing Drug-Eluting Balloon Education
 Advanced Workshop CLI and Revascularization Management, November 2023, Abano Terme, Italy
Advanced Workshop CLI and Revascularization Management, November 2023, Abano Terme, Italy
 How to maximize DEB benefits: a case based practical guide in lower limbs,CIRSE congress, September 2023, Denmark
How to maximize DEB benefits: a case based practical guide in lower limbs,CIRSE congress, September 2023, Denmark
 How I treat CLTI patients with novel Sirolimus DEB: DEB session, PAIRS 2024 conference, February 2024, UAE
How I treat CLTI patients with novel Sirolimus DEB: DEB session, PAIRS 2024 conference, February 2024, UAE
 Managing CLI patients: From vessel preparation techniques to treatment with sirolimus DEBs, DEB session, LINC congress, May 2024, Germany
Managing CLI patients: From vessel preparation techniques to treatment with sirolimus DEBs, DEB session, LINC congress, May 2024, Germany

Empowering healthcare practitioners through professional education initiatives, workshops and webinars
Customer Service and Ordering Information
For country-specific contact details, please see this page.