
IKAZUCHI ZERO Semi-Compliant PTCA Balloon
IKAZUCHI ZERO™ Semi-Compliant PTCA Balloon is engineered to facilitate the crossing including for complex lesion.*
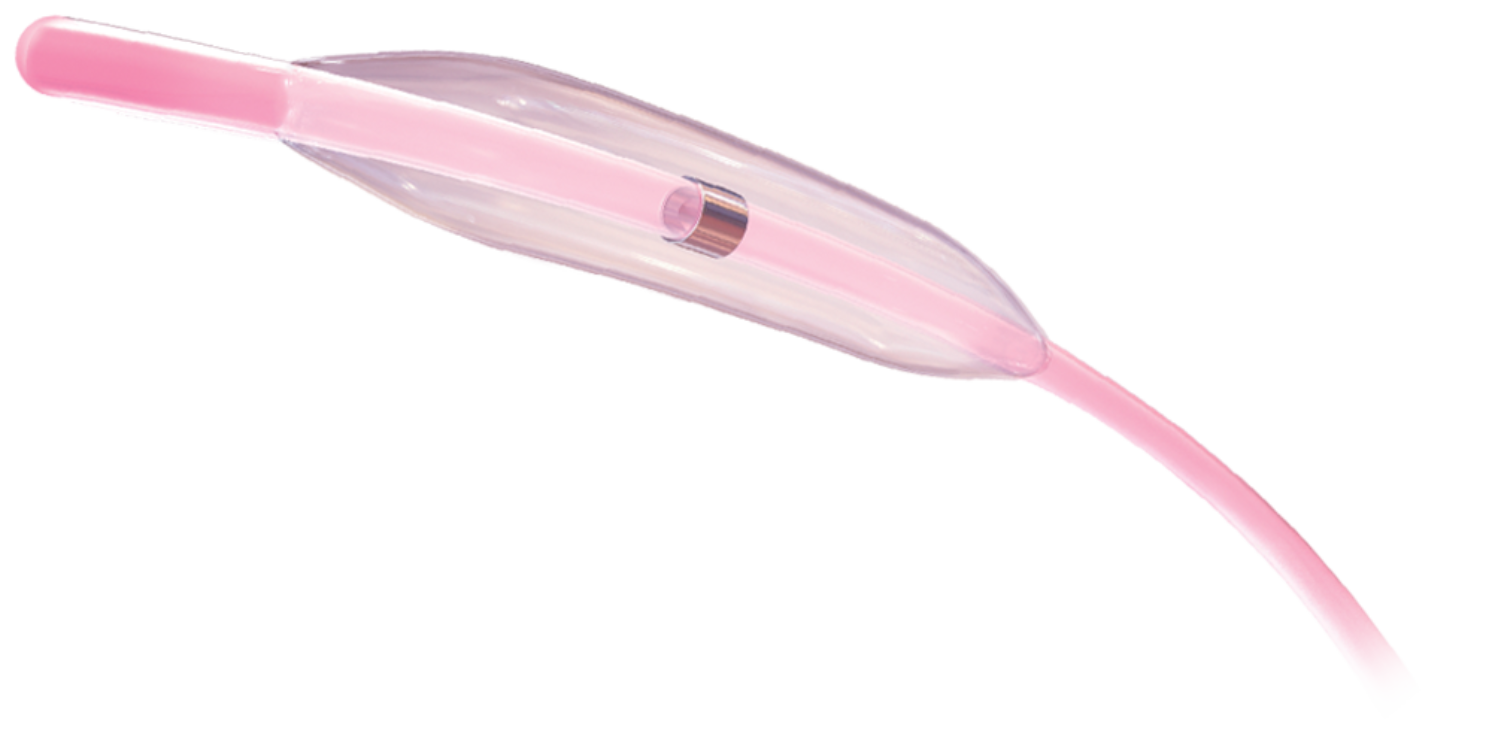
Product Description
Engineered to facilitate the crossing including for complex lesions**
- Low-profile 1 mm diameter pre-dilatation balloon available
- Minimized entry and balloon profile with optimized pushability and tip flexibility
- TR2 hydrophilic coating improves lubricity, thereby reducing resistance
- Manufactured by Kaneka Corporation, one of Japan’s leading providers of interventional device
* This website contains information on products for a wide range of countries. As a result, it may contain information about products not available in your country. For more information, please contact your local Cordis representative.
** Challenging lesions such as calcified or tortuous lesions may not be crossed with this catheter. The physician in charge of the procedure should determine whether this catheter is applicable based on his/her past experiences.
IKAZUCHI ZERO is a trademark of Kaneka Corporation, the legal manufacturer, and distributed by Cordis.
IKAZUCHI ZERO™ & RAIDEN3™ PTCA Balloons
Resources
Please refer to Cordis Product Catalogue for complete product information.
IFU & Safety
Please refer to the Instructions for Use for complete information, including indications, precautions, warnings, and potential adverse events.
To request product specific Summary of Safety and Clinical Performance (SSCP), please email CordisMedInfo@cordis.com.
Customer Service and Ordering Information
For country-specific contact details, please see this page.

