
TEMPO AQUA Diagnostic Catheter
TEMPO AQUA™ Hydrophilic Coated Angiographic Catheter has a truly bonded hydrophilic coating, that enables a smooth passage through the vessel.*
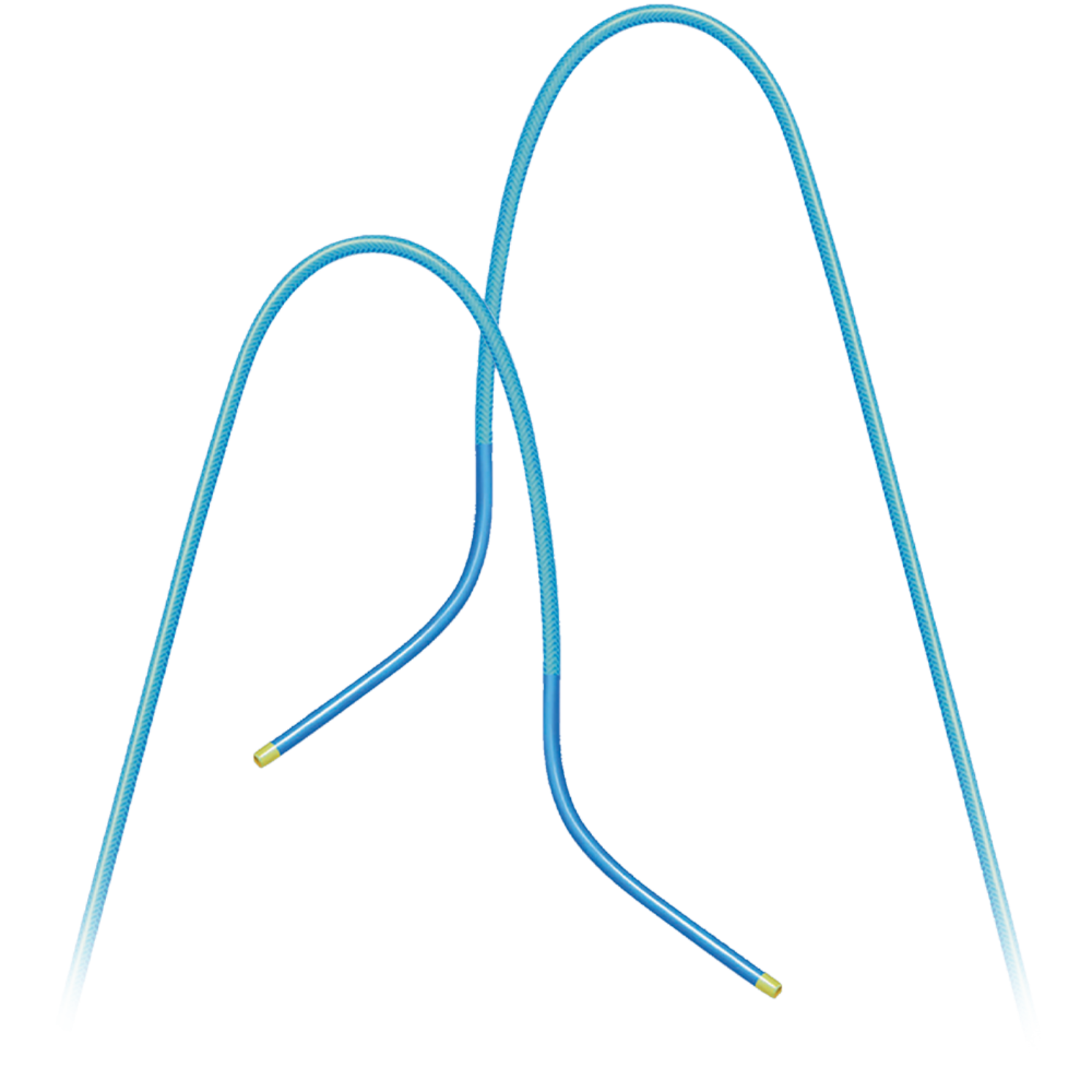
Product Description
Cordis has an extensive line of diagnostic catheters both for selective and flush applications. TEMPO™ and TEMPO AQUA catheters have a nylon body and the SUPERTORQUE™ Catheter line has a polyurethane shaft.
Flush catheters are available: braided and non-braided, straight and pigtail versions, extensive range of lengths and French-sizes.
All the Cordis selective catheters have a unique braiding that provides excellent torque control. TEMPO AQUA Catheter has a truly bonded hydrophilic coating, that enables a smooth passage through the vessel. All commonly used shapes are available in different French-sizes.
TEMPO AQUA Catheters offer:
Precise control and sturdy support
High-strength nylon construction and braided shaft design give strength and flexibility for superb torque response and maximum tactile control.
Enhanced tip radiopacity
An extended, tungsten-filled neon tip, with superb visualization for rapid and precise catheter placement. Less need for contrast injection.
Flexible tip
Patented soft design and long non-braided distal end increases tip flexibility, helps to reduce the risk of vessel trauma and promotes patient comfort.
Smoother delivery
Hydrophilic SLX™ Coating minimizes friction between the catheter and the vessel wall for rapid and smooth vessel engagement.
* This website contains information on products for a wide range of countries. As a result, it may contain information about products not available in your country. For more information, please contact your local Cordis representative.
Resources
Please refer to Cordis Product Catalogue for complete product information.
IFU & Safety
Please refer to the Instructions for Use for complete information, including indications, precautions, warnings, and potential adverse events.
To request product specific Summary of Safety and Clinical Performance (SSCP), please email CordisMedInfo@cordis.com.
Customer Service and Ordering Information
For country-specific contact details, please see this page.

