
MYNXGRIP Vascular Closure Device
Security of mechanical closure. Safety of an extravascular sealant.
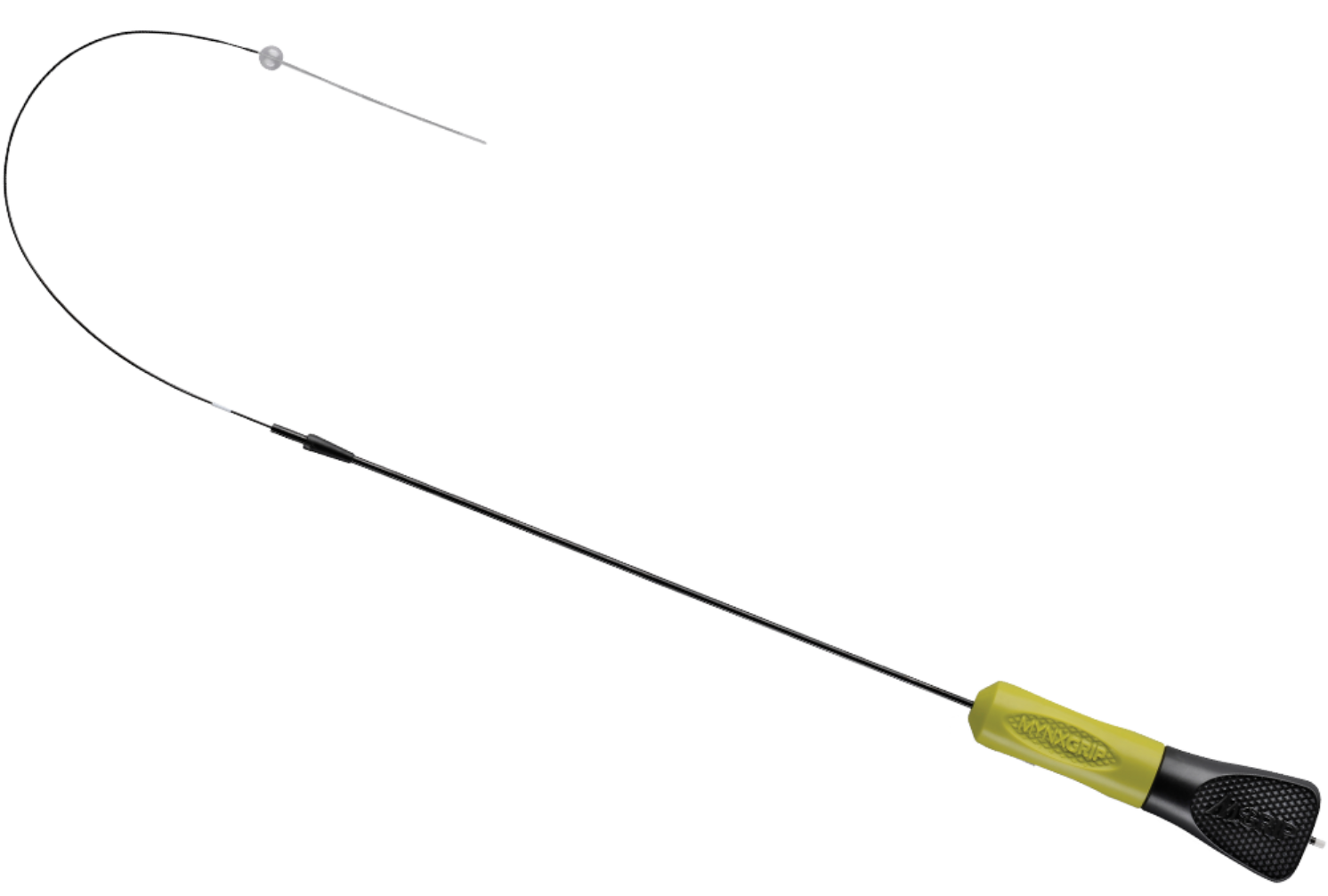
Product Description
The MYNXGRIP® Vascular Closure Device (VCD) achieves secure extravascular closure by utilizing the Grip sealant, which actively adheres to the artery or vein. The MYNXGRIP VCD is also indicated to close femoral venous access sites utilizing a 5F, 6F, or 7F procedural sheath.
The MYNXGRIP VCD provides secure mechanical closure with the safety of an extravascular sealant. The MYNXGRIP VCD contains the proprietary Grip sealant which actively adheres to and seals the arteriotomy or venotomy while expanding to fill the tissue tract. The MYNXGRIP VCD offers a patient-friendly closure option with no cinching, suturing, or metal implants. The Grip sealant dissolves within 30 days leaving nothing permanently behind but a healed artery.
Why Compromise?
The MYNXGRIP VCD provides secure vascular closure without the trade-offs. Built on the proven MYNX Family of Vascular Closure products, the MYNXGRIP VCD offers the security of mechanical closure combined with the safety of an extravascular sealant. The MYNXGRIP VCD offers a patient-friendly closure option with no sutures, clamping, or metal implants and dissolves within 30 days leaving nothing behind but a healed artery.
Product Characteristics
Scroll, sort or search for the particular MYNXGRIP Vascular Closure Device's part number of interest.
| SKU | Product Description | Sheath Compatibility (F) | Qty/box | Where Used/Indication |
|---|---|---|---|---|
| MX6721 | MYNXGRIP Vascular Closure Device 6/7F | 6/7 | 10 | Femoral artery/vein |
| MX5021 | MYNXGRIP Vascular Closure Device 5F | 5 | 10 | Femoral artery/vein |
References
- Mynx Vascular Closure Device Achieves Reliable Closure and Hemostasis of Percutaneous Transfemoral Venous Access in a Porcine Vascular Model S. Sanjay Srivatsa, MBBChir1; Arjun Srivatsa1; Taylor A. Spangler, DVM, DACVP2. The Journal of Invasive Cardiology. February 2015
- The MYNXGRIP® Vascular Closure Device Hoang Minh Thai, MD, FACC, FSCAI, and Barry S. Weinstock, MD. Endovascular Today. April 2012.
- Successful Reduction of Surgeries Secondary to Arterial Access Site Complications A Retrospective Review at a Single-Center with an Extravascular Closure Device. Noor S, Meyers S, Curl R. Vascular and Endovascular Surgery. May 2010;44(5):345-9.
- A Prospective Randomized Single-blind trial of Patient Comfort Following Vessel Closure Extravascular Synthetic Sealant Closure Provides Less Pain than a Self-tightening Suture Vascular Compression Device. Fargen KM, Hoh BL, Mocco J. J Neurointerv Surg. 2011;3(3):219-23
- Mynx Vascular Closure Device Early Ambulation Study Vikranth R. Gongidi, DO; Ahsan Jafir, DO; and Vijay Verma, MD, FACC, FSCAI. Endovascular Today. October 2013
- Occurrence of angiographic femoral artery complications after vascular closure with Mynx and AngioSeal Fargen KM, Velat GJ, Lawson MF, et al. Journal of Neurolnterventional Surgery. 2013;5(2):161-4
- Use of Vascular Closure Device After Access of Common Femoral Artery Through an Existing Stent George JC. Vascular Disease Management. 2012;9(5):E68-E70.
- Two Years of Extravascular Closure With the Mynx® Vascular Closure Device The Baptist Memorial Hospital experience revisited. David Wolford, MD, FACC. Endovascular Today. August 2010.
- Mynx Closure in a Patient Treated for Chronic Total Occlusion of the Iliac Artery with Multiple Sheath Exchanges. A Case Review. Michael S. Fenster, MD. TCTMD. May 2009.
- Why Extravascular Closure? The Baptist Memorial Hospital Experience with the Mynx Vascular Closure Device David Wolford, MD, FACC. Endovascular Today. August 2009.
- The Safety and Efficacy of an Extravascular, Water-Soluble Sealant for Vascular Closure Initial Clinical Results for Mynx. D. Scheinert MD et al. Catheterization and Cardiovascular Interventions. November 2007.
IFU
Please refer to the Instructions for Use for complete information, including indications, precautions, warnings, and potential adverse events.
Customer Service and Ordering Information
In the United States, email us your question or order, or call us at 800.327.7714.

