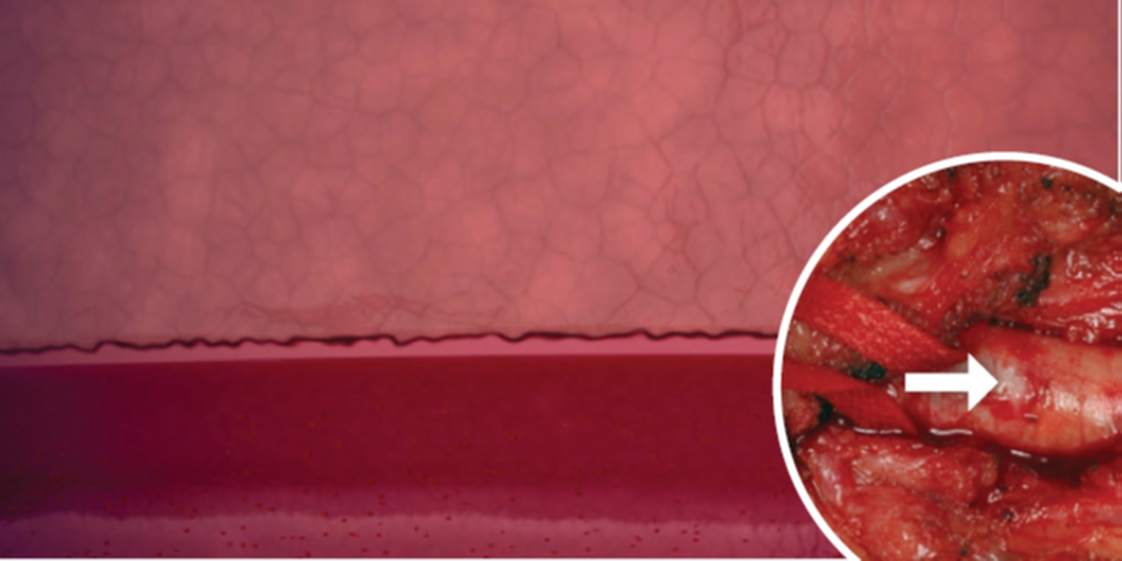
MYNX CONTROL VENOUS Vascular Closure Device
Move Foward with MYNX CONTROL™ VENOUS Vascular Closure Device (VCD), achieving rapid hemostasis.
Request Additional Information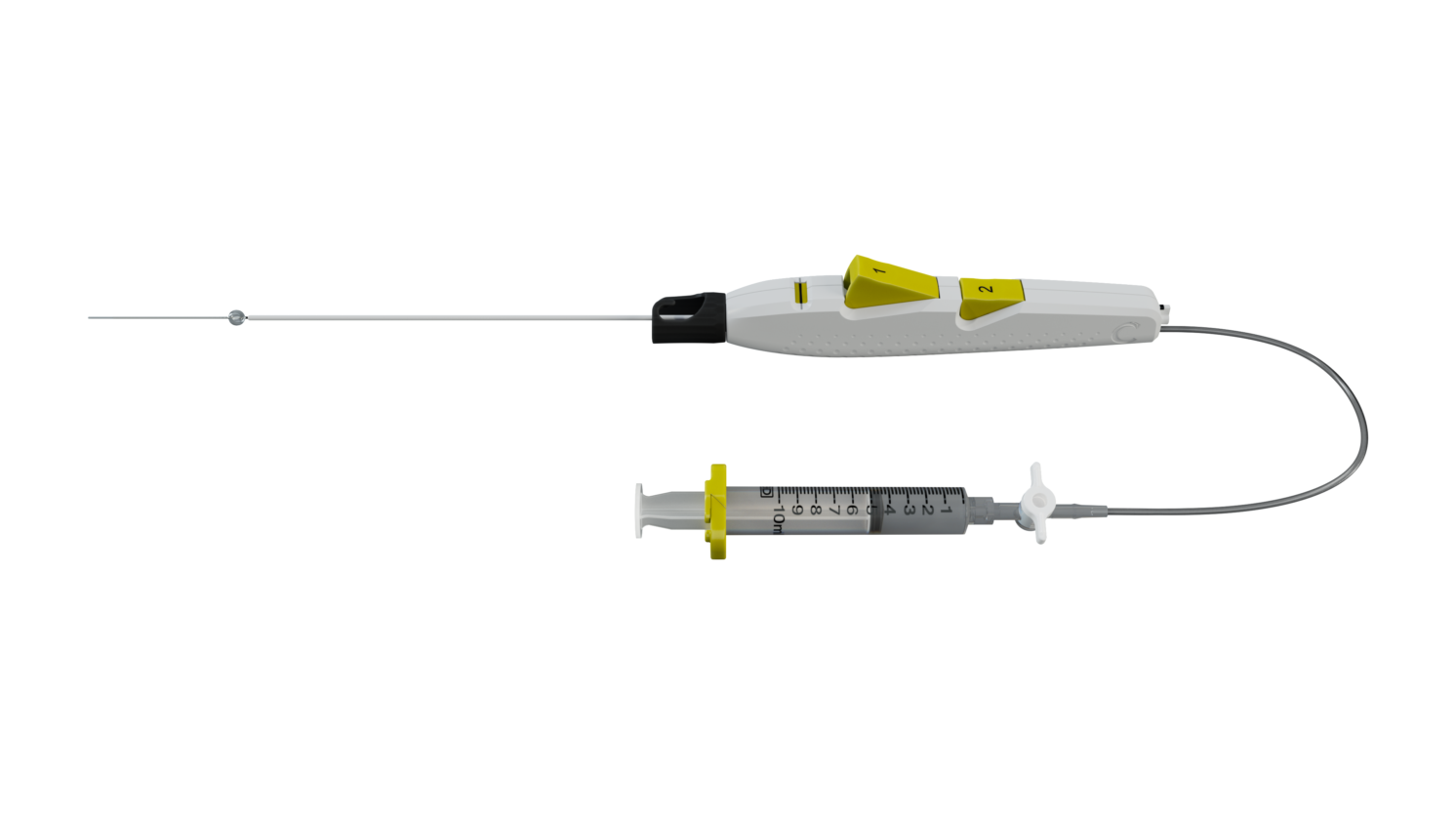
Move Forward With MYNX
DESIGNED FOR EASY AND CONFIDENT DEPLOYMENT
MYNX CONTROL VENOUS VCD is designed to pair procedural simplicity with enhanced safety & reliability.
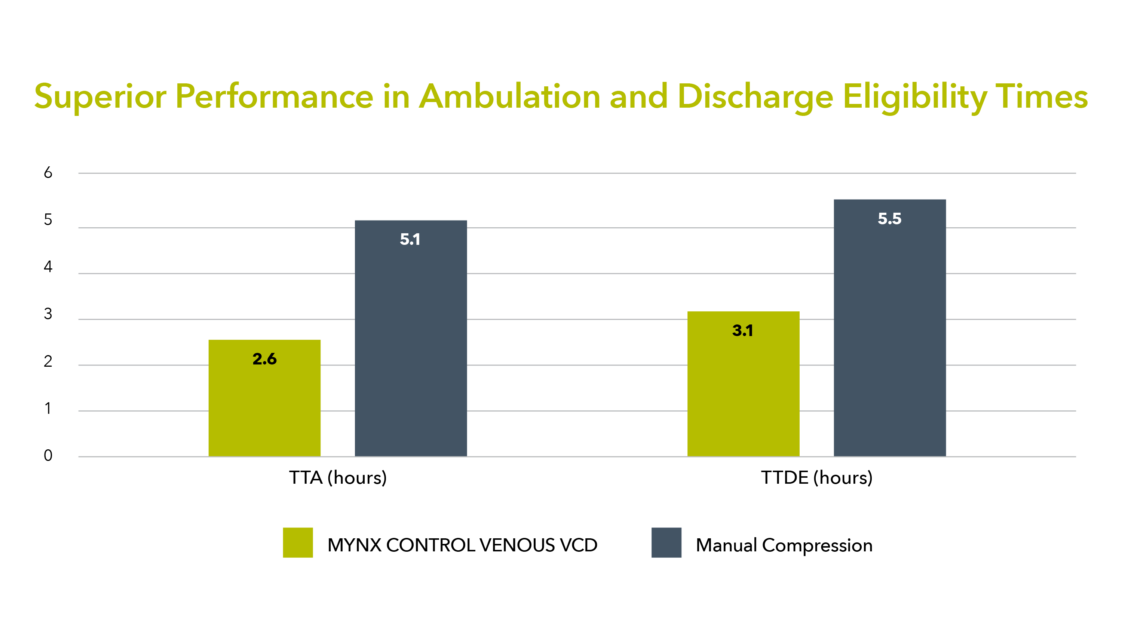
ACHIEVES RAPID TIME TO HEMOSTASIS
Save valuable staff time and move your patients to a quick recovery and on average eligible to discharge within 3 hours1-3.
RESORBS 3X FASTER THAN COLLAGEN SEALANTS
Quick and consistent resorption within 30 days, leaving nothing behind to impede future re-access1,4.
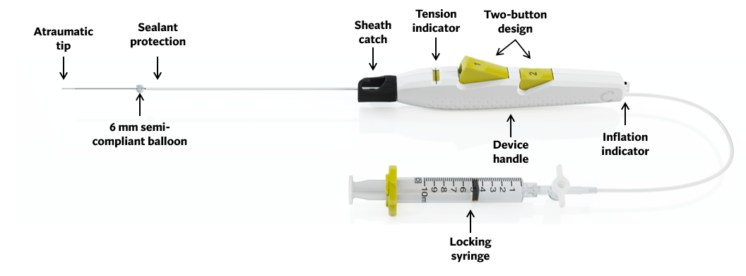
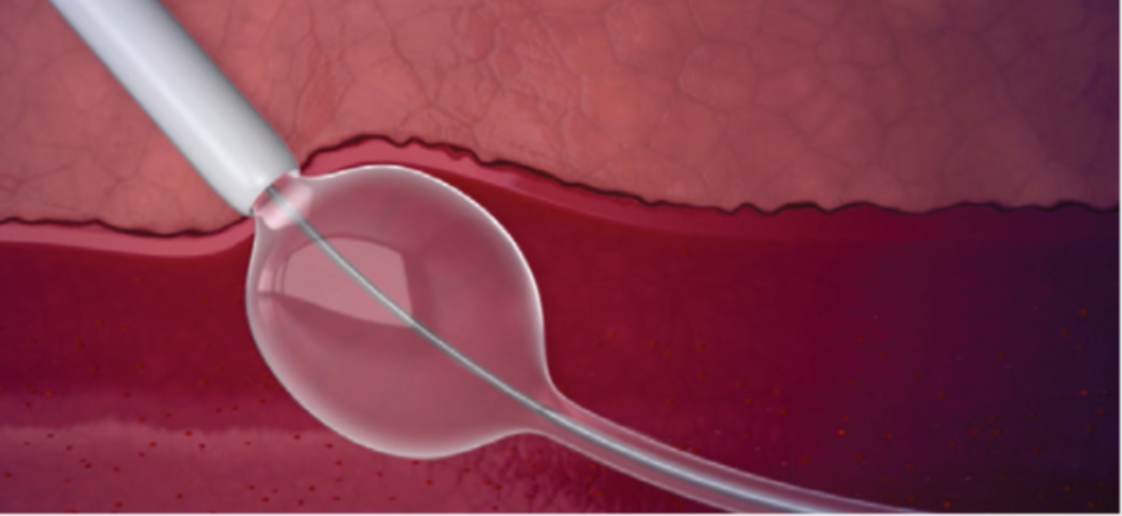
Designed For Easy and Confident Deployment
Sealant Protection
- Minimizes sealant expansion inside cannula
- Facilitates smooth deployment
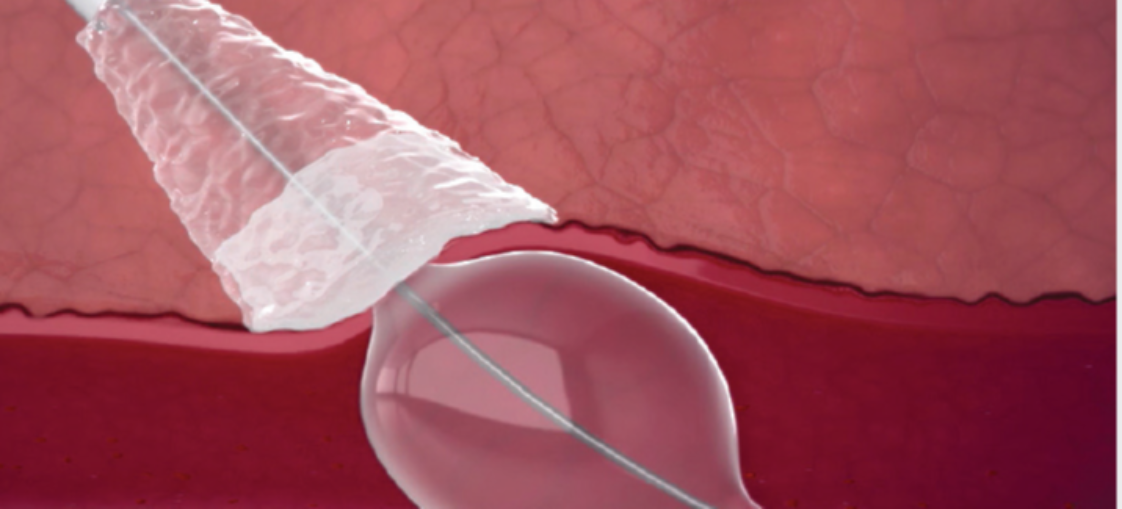
Sheath Catch
- Sized to accommodate 6F-12F sheath ID (Inner Diameter)
(Note: Use only with a standard sheath introducer with up to 12 cm effective length. Incompatible with Cook Check-Flo™ Performer™ Introducer)
Tension Indicator
- Prevents vessel tenting allowing for a more consistent seal
- Provides confirmation of correct deployment position
Two-Button Design
- Ergonomic handle and buttons for a simple and easy deployment
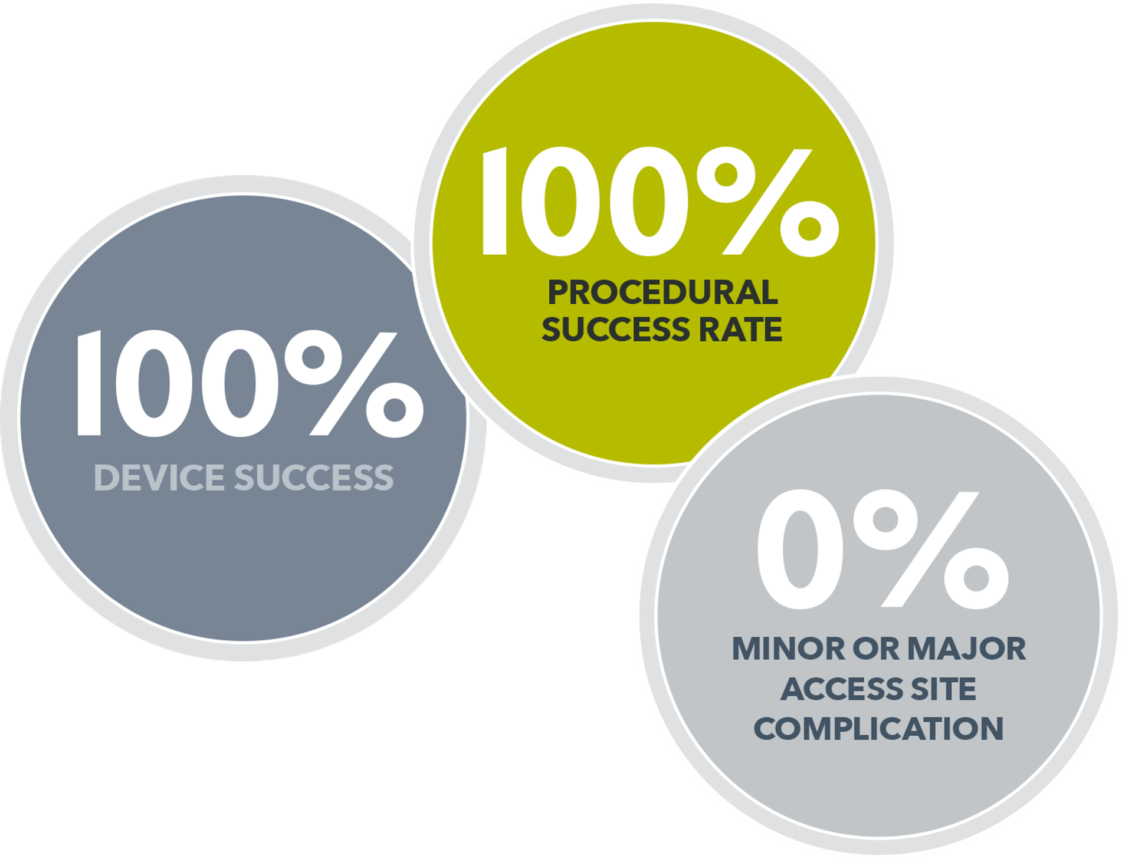
Clinical Highlights: ReliaSeal Study Recap
MYNX CONTROL VENOUS ReliaSeal Study Recap
This is a brief interview with Dr. Gupta, Saint Luke's Cardiovascular Consultants, Kansas City, MO after his Abstract Presentation at TCT 2024 presenting the ReliaSeal Study results.
MYNX CONTROL VENOUS VCD Animation
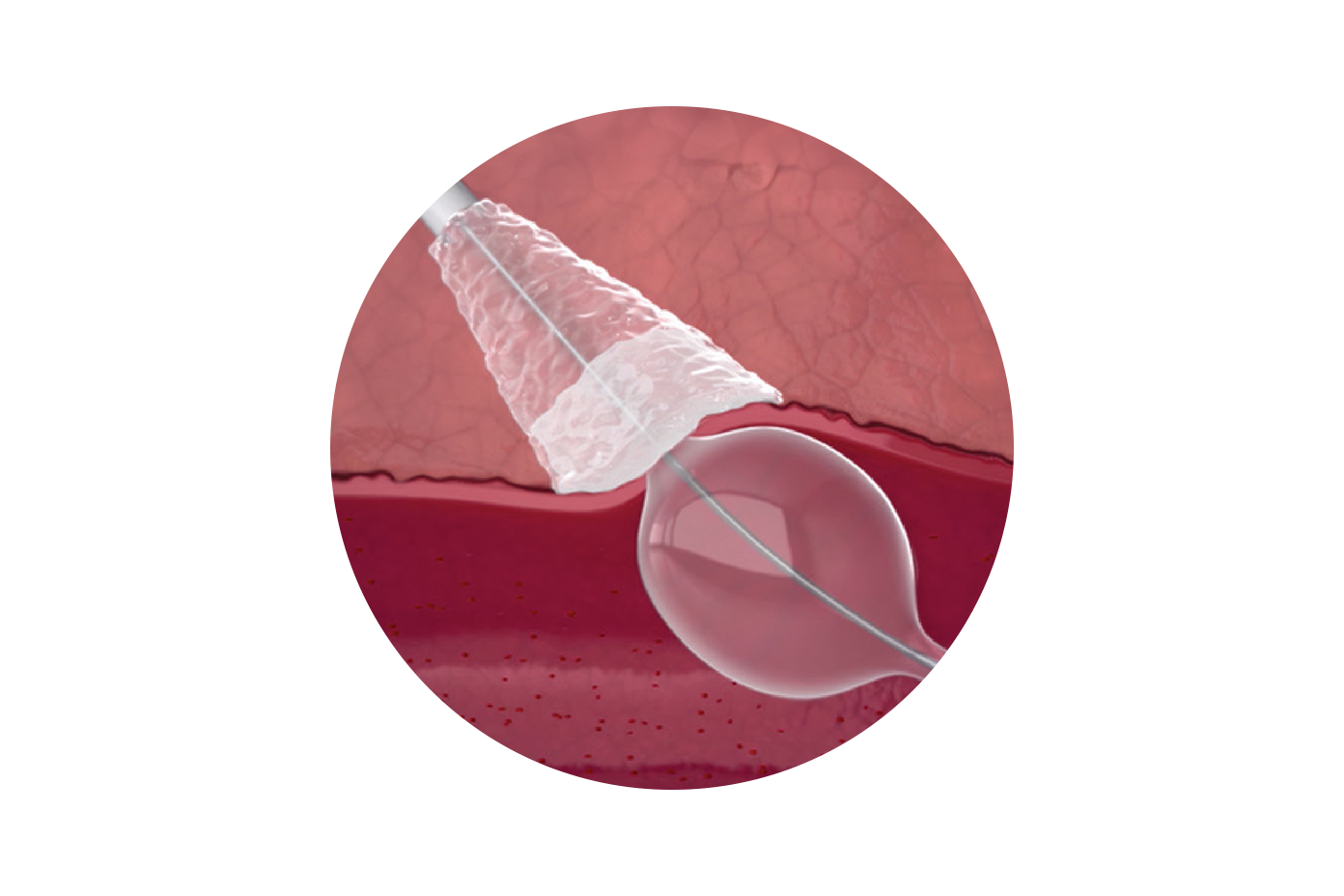
See how the MYNX® CONTROL VENOUS Vascular Closure Device simplifies large-bore venous closure with a bioresorbable, extravascular seal that preserves vessel integrity. This animation highlights its controlled, step-by-step deployment for consistent hemostasis, purpose-built design for interventional specialists, and efficient performance to support smoother workflows and better patient outcomes.
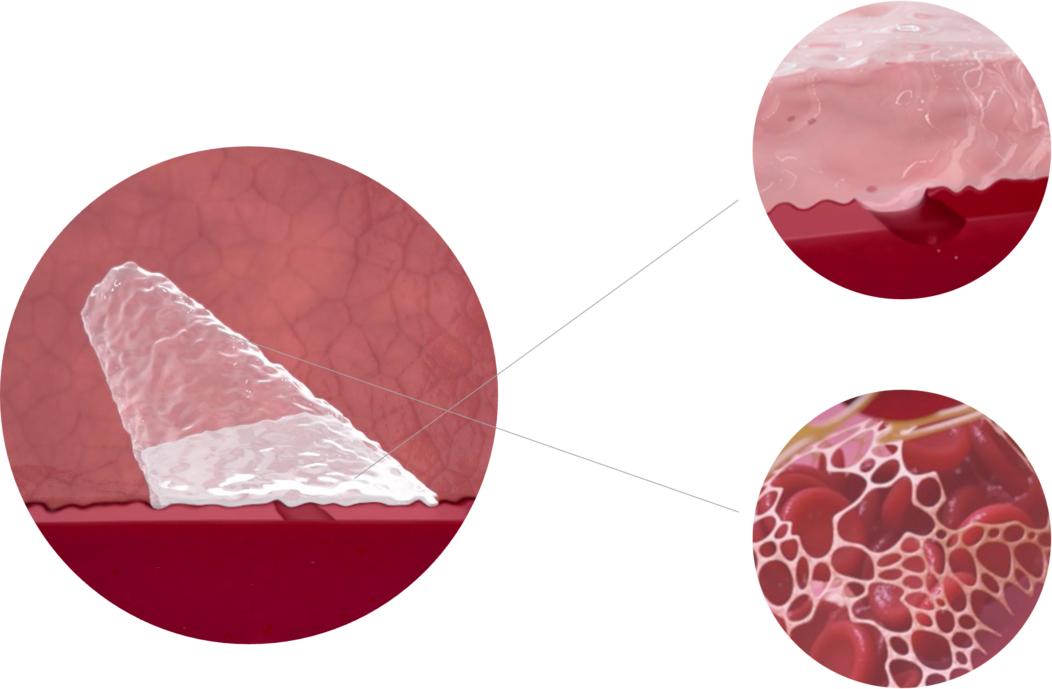
Resorbs 3X Faster Than Collagen Sealants
GRIP TECHNOLOGY™ is made of a hydrophilic, bio-inert polyethylene glycol (PEG)1,4.
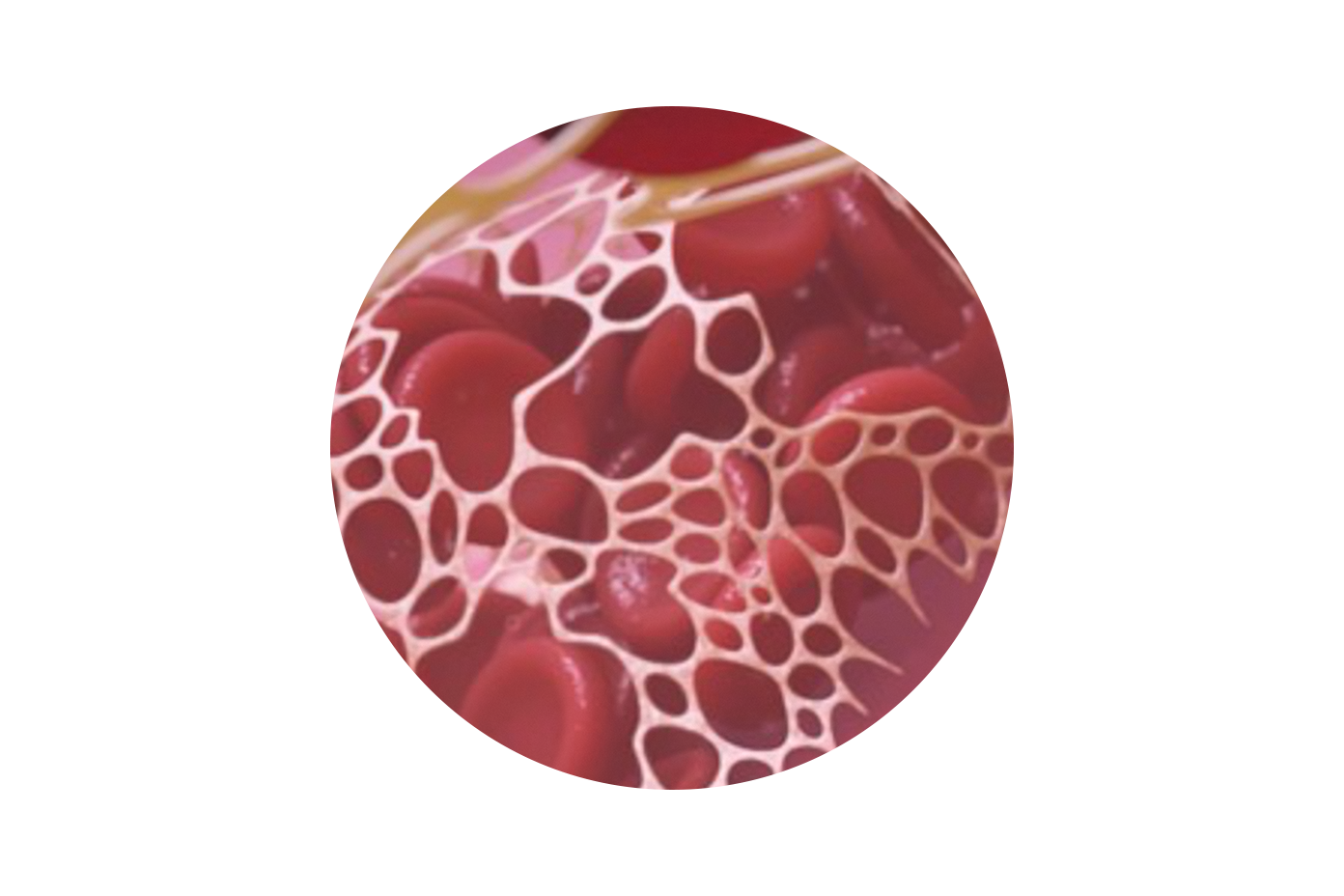
Promotes Natural Vessel Healing
Helps to minimize access site infections
Quick and consistent resorption within 30 days1

Product Characteristics
Scroll, sort or search for the particular MYNX CONTROL VENOUS Vascular Closure Device's part number of interest.
| SKU | Product Description | Sheath Compatibility (F) | Qty/box | Where Used/Indication |
|---|---|---|---|---|
| MX61260 | MYNX CONTROL VENOUS Vascular Closure Device 6-12 | 6 - 12 | 10 | Femoral vein |
- 1. Cordis 2024 Data on File.
- 2. Natale A, Mohanty S, Liu PY, et al. Venous Vascular Closure System Versus Manual Compression Following Multiple Access Electrophysiology Procedures: The AMBULATE Trial. JACC Clin Electrophysiol. 2020;6(1):111-124. doi:10.1016/j.jacep.2019.08.013.
- 3. Mohammed M, Ramirez R, Steinhaus DA, et al. Comparative outcomes of vascular access closure methods following atrial fibrillation/flutter catheter ablation: insights from VAscular Closure for Cardiac Ablation Registry. J Interv Card Electrophysiol. 2022;64(2):301-310.
doi:10.1007/s10840-021-00981-5.
- 4. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. VASCADE Vascular Closure System (VCS) P120016 approval letter, January 31, 2013. https://www.accessdata.fda.gov/cdrh_docs/pdf12/P120016b.pdf.
IFU
Please refer to the Instructions for Use for complete information, including indications, precautions, warnings, and potential adverse events.
Customer Service and Ordering Information
In the United States, email us your question or order, or call us at 800.327.7714.