
IKAZUCHI ZERO Semi-Compliant PTCA Balloon
Engineered to facilitate crossing lesions, including those that are more complex.*
* This website contains information on products for a wide range of countries. As a result, it may contain information about products not available in your country. For more information, please contact your local Cordis representative.
Product Description
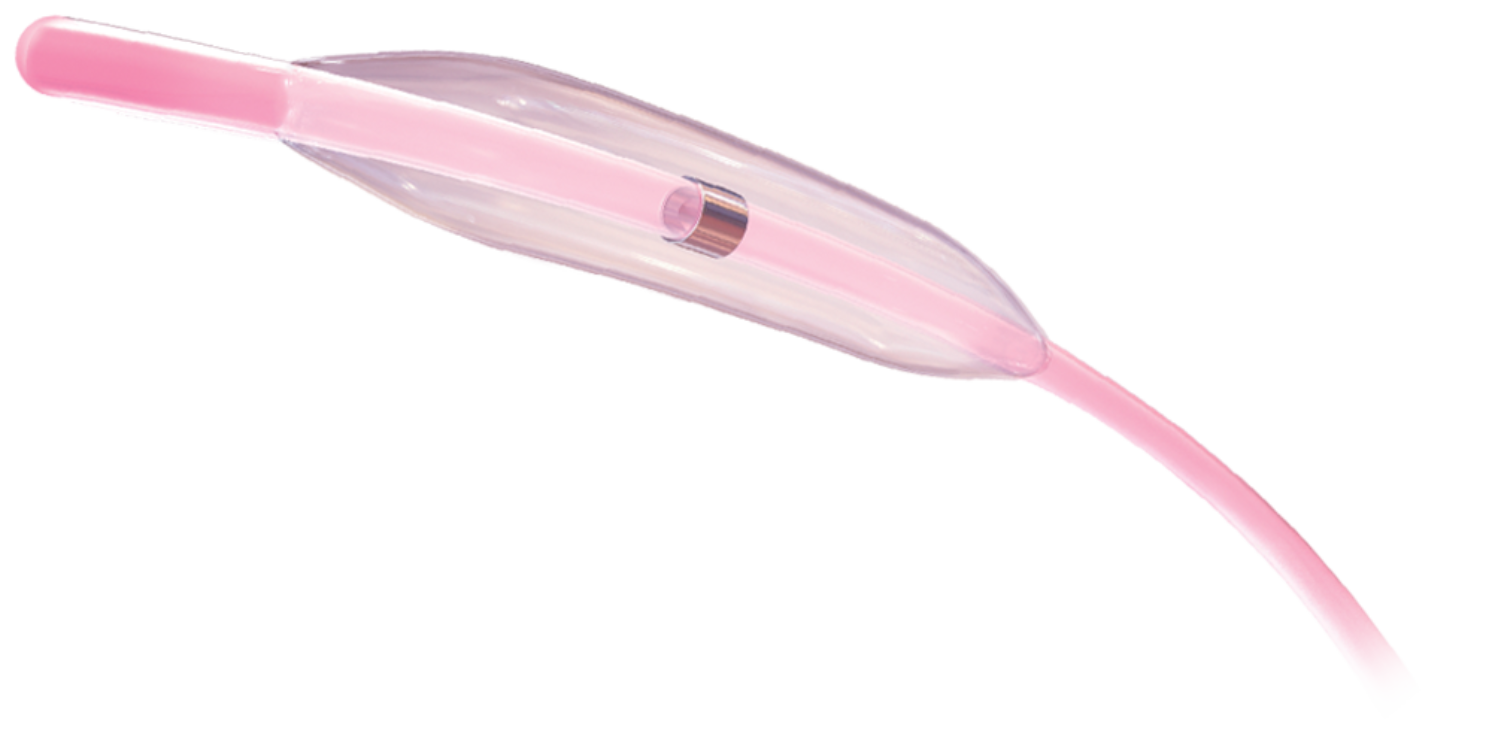
The IKAZUCHI ZERO™ Semi-Compliant PTCA Balloon is engineered to facilitate crossing lesions, including those that are more complex, including for complex lesions*
- Featuring the lowest available profile**
- Minimized entry and balloon profile with optimized pushability and tip flexibility
- Next generation TR2 hydrophilic coating improves lubricity, thereby reducing resistance
- Manufactured by Kaneka Corporation, one of Japan’s leading providers of interventional devices
* Challenging lesions such as calcified or tortuous lesions may not be crossed with this catheter. The physician in charge of the procedure should determine whether this catheter is applicable based on his/her past experiences.
**Kaneka Data on File.
IKAZUCHI Zero Balloons are manufactured by Kaneka Corporation and distributed by Cordis.
IFU
Please refer to the Indications for Use for complete information, including indications, precautions, warnings, and potential adverse events.

